EXCELLENCE IN CLINICAL ANALYSIS
Demonstrate the reliability of your results
The Proficiency Testing (EP), also known as External Quality Control (EQC), is an effective quality control tool for determining the analytical performance of the laboratory and a necessary requirement for laboratory accreditation processes (ISO 15189, ISO 17025, PALC-SBPC/ML, DICQ-SBAC, etc.) and regulatory bodies.
Controllab Catalog
Get to the broad portfolio, with more than 3,500 tests covered by quality control and sign up to highlight the quality of the tests performed in your routine.
QUALITY


PERFORMANCE
Learn more about the benefits
Standardize the analytical stage against the market
Assess the efficiency of internal control and ensure its suitability for specific methods
Evaluate and monitor the performance of laboratories in specific tests
Identify hits and compliance
Determine the performance characteristics of already established methods and/or new methods and technologies
Provide corrective/preventive actions
In summary, in addition to assessing technical quality, the Proficiency Testing is:
Mandatory by regulatory bodies
Accreditation process and bidding prerequisites
Requirements for accreditation with the main health insurers
Differential against to the competition
ACCREDITATIONS AND
RECOGNITIONS
Continuous improvement, based on quality and reliability, has given Controllab some recognition for the Proficiency Testing:
According to the scopes
published in www.inmetro.gov.br
How can Proficiency Testing
help in your laboratory?
ASSIST THE LABORATORY ROUTINE
Assessments are continuous, with regular intervals, annual targets and multiple items in varying concentrations. These assessments are the result of statistical studies and analysis by experts, whose reports commonly point out errors, possible causes and considerations on the overall performance of the participants. These actions take place so that each one can compare their performance with the others, at the moment and over time.
How it works?
Learn more about how Controllab Proficiency Testing works by clicking on the steps below.
Service
Proficiency Testing with rounds at regular and continuous intervals, annual targets and multiple items with unknown results for analysis. Structured in ABNT NBR ISO/IEC 17043.
Participation is simple and carried out in 3 stages:
Proficiency Testing programs are organized in the form of rounds, grouped in an annual calendar. In these rounds, a set of items (samples) with unknown results are sent simultaneously to the participating laboratories in order to carry out the relevant analyzes. For virtual items, this step is suppressed.
Testing by the participating laboratory shall be performed in a manner similar to the routine of testing. Subsequently, Controllab should be provided with information such as: results, methods, equipment, reactives, among others, used to perform the analyses.
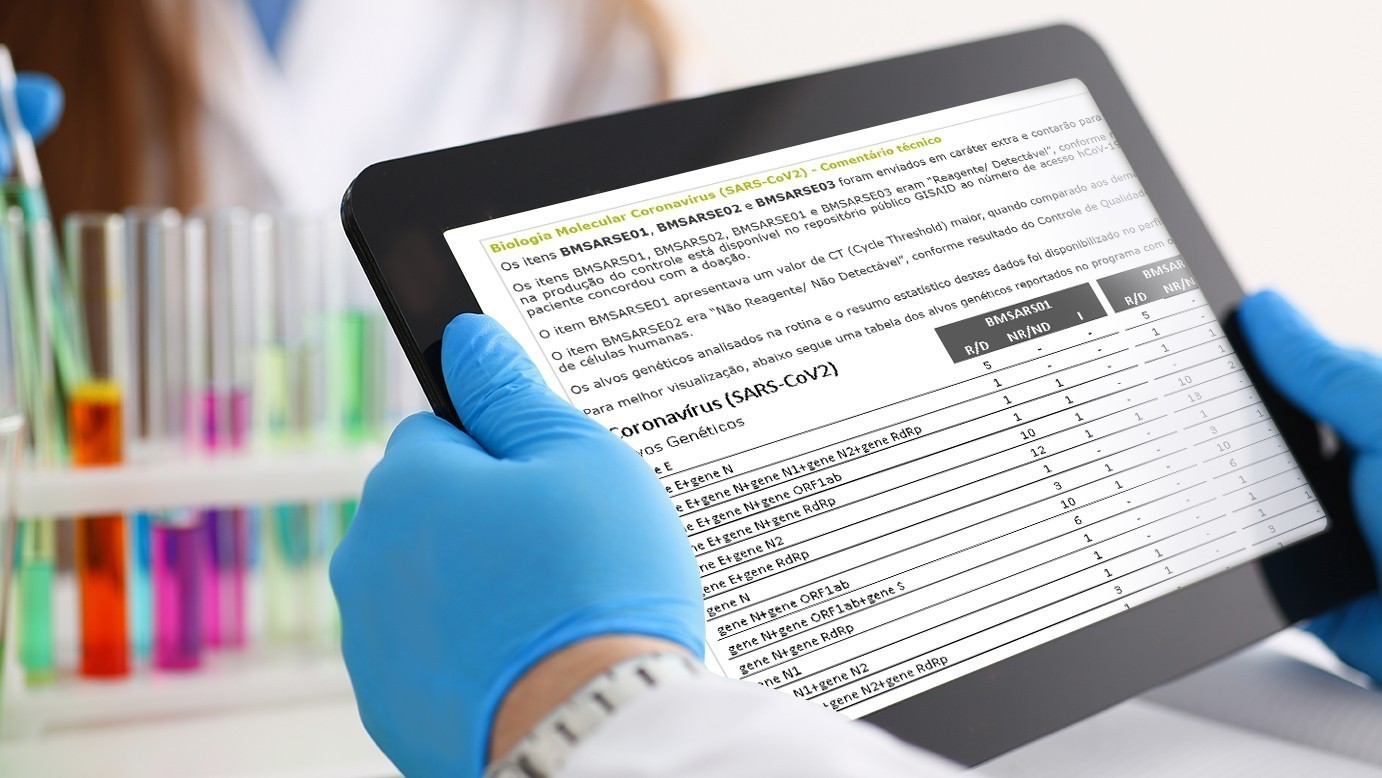
After receiving the information, Controllab issues individual laboratory evaluation reports, global statistical reports with technical and market information and where relevant, technical considerations and reports of Controllab’s scientific advisors.
Test Items
Virtual Microscopy
Participantes
Organizações de diferentes portes comparam suas análises com laboratórios consolidados em excelência analítica.
Abrangência
Rounds
Sending samples that simulate routine testing. The laboratory reports the results online together with information from the analytical system for Controllab to perform the comparison and evaluation.
Online System
Administration
Evaluation
Comparison based on the most specific groups and limits applied according to statistical studies based on ABNT NBR 13528.
Results Evaluation
Reports
Individuals with the performance of the round and graphs of the Deviation Index and Z Index; global market with technical and marketing information and continuous performance over the period.
Evaluation Report (Individual)
Results Profile (Public)
Management
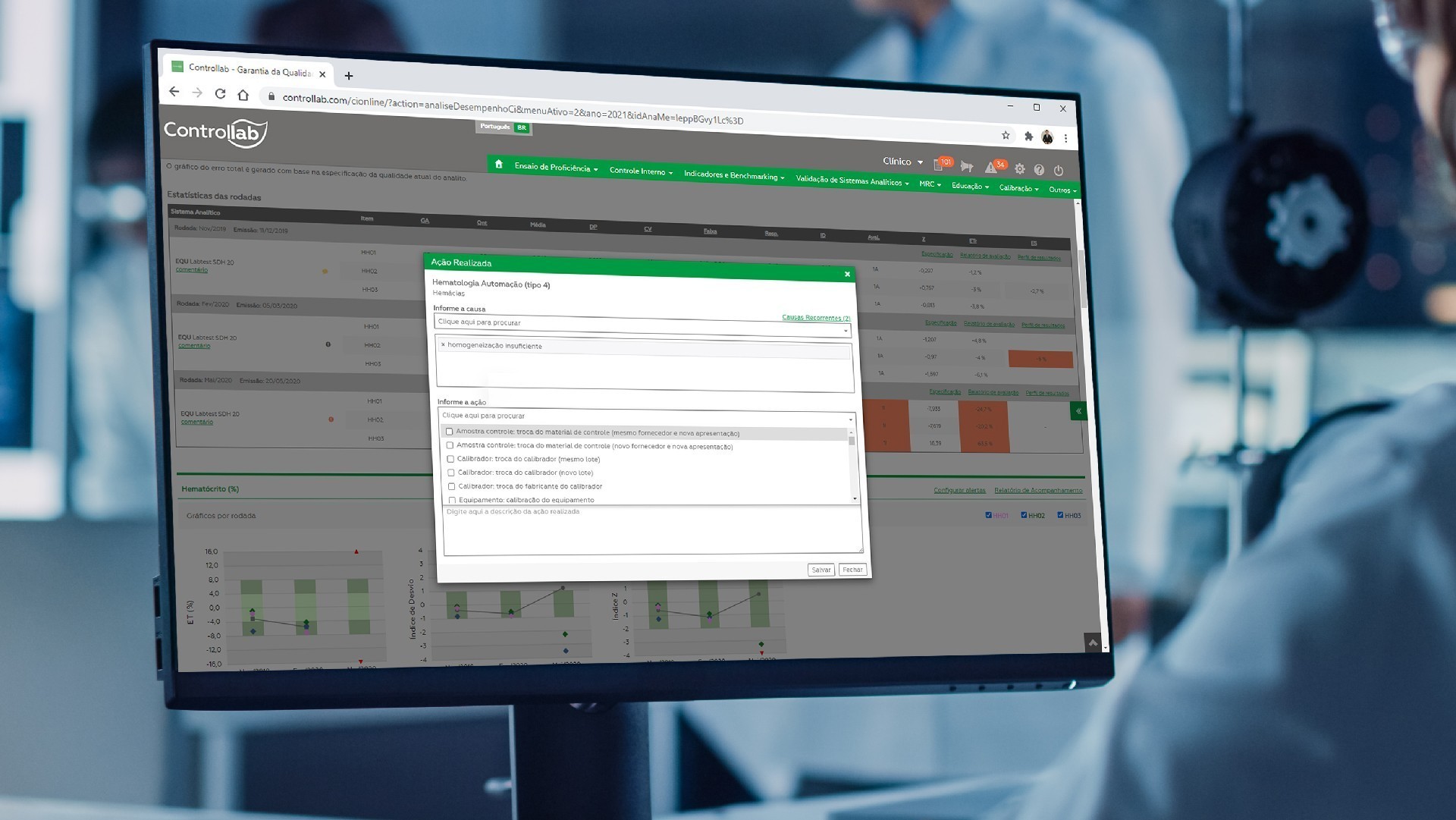
Management center with summary of analytical performance and indicators to achieve the goals of excellence in analysis.
Analytical performance management
How it works?
Learn more about how Controllab Proficiency Testing works by clicking on the steps below.
Service
Proficiency Testing with rounds at regular and continuous intervals, annual targets and multiple items with unknown results for analysis. Structured in ABNT NBR ISO/IEC 17043.
Participants
Organizations of different sizes compare their analysis with consolidated laboratories in analytical excellence.
Rounds
Sending samples that simulate routine testing. The laboratory reports the results online together with information from the analytical system for Controllab to perform the comparison and evaluation.
Evaluation
Comparison based on the most specific groups and limits applied according to statistical studies based on ABNT NBR 13528.
Reports
Individuals with the performance of the round and graphs of the Deviation Index and Z Index; global market with technical and marketing information and continuous performance over the period.
Management
Management center with summary of analytical performance and indicators to achieve the goals of excellence in analysis.
The delivery of health services linked to quality is a constant concern. For this reason, the Proficiency Testing assists laboratories so that they can guarantee quality delivery, in addition to allowing continuous improvement in their processes. With continuous participation in the Proficiency Testing, the laboratory demonstrates competence to perform a certain test or measurement.
Promotion
Continuous participation is an investment in quality and can be disclosed through the registration certificate, disclosure seal and certificates of proficiency.
The certificate of enrollment and the quality control seal are available after 3 months of participation in the program. Annually, the laboratory receives the certificate of proficiency for the tests that obtained continuous participation and that reached the minimum level of performance.
These documents demonstrate the commitment of the laboratory to proficiency testing and support the marketing of the laboratory, favoring the transparency of information and valuing participation in the service.
They have a format that gives more trust and credibility to customers, doctors, patients, auditors and other stakeholders in the organization.
The Clinical Proficiency Testing has the seal of the Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC/ML).
The veterinary program is supported by the Brazilian Society of Veterinary Medicine (SBMV).