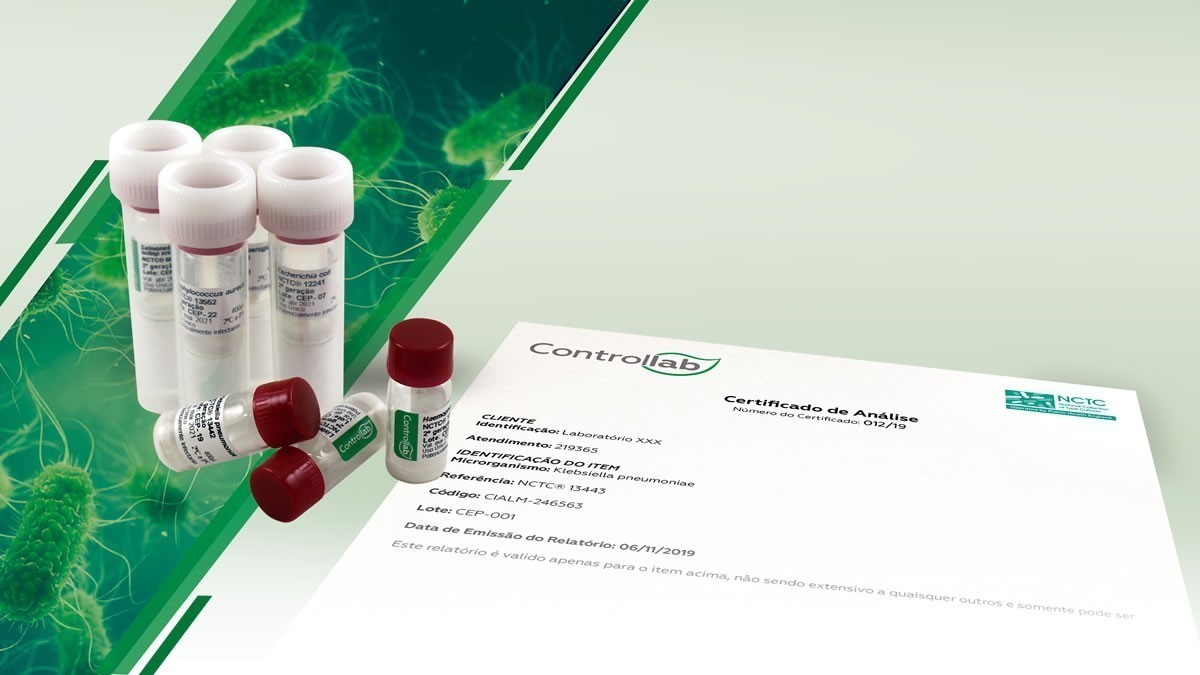
To facilitate the access of laboratories to the reference strains, Controllab makes available to Brazil and Latin America the most renowned collection of cultures in Europe: NCTC
In the routine of the analysis laboratories, the reference strains are fundamental for the quality control of the inputs used in the clinical and veterinary diagnostic exams, in the microbiological analysis of food, water, pharmaceutical products and the environment. They are also of great importance for carrying out studies to validate analytical methods and determine measurement uncertainty.
In its continuous search to provide the best solutions in laboratory quality control, Controllab has partnered with PHE – Public Health England. This initiative made it possible for Brazil and other Latin American countries to have immediate access to the oldest bacterial collection in the world – NCTC (National Collection of Type Cultures) – founded in 1920.
There are more than 60 certified strains available for prompt delivery and in the quantity defined by the laboratory. They help laboratories to comply with NBR ISO/IEC 17025 requirements regarding the quality control of results.
Strains are used in the main validations: test for detection of microorganisms, alternative methods, new equipment, microbiological inputs (culture media), etc. They give more confidence and credibility to quality control, facilitating the process of meeting standards, laws, audits, accreditations and inspections.
Having access to lyophilized and certified strains, optimizes maintenance resources in the laboratory. With them, the laboratory only reconstitutes the inoculum, performs the sowing and incubation.
“Most analysis laboratories in Brazil do not have a freezer at -70 ° C, which is necessary for the maintenance of reference strains for an extended period of time. In particular, nutritionally demanding microorganisms such as Streptococcus pneumoniae, Haemophilus influenzae, Neisseria gonorrhoeae and Campylobacter do not survive freezing at -20 ° C. In addition, the successive repetition of reference strains can result in the alteration or loss of their characteristics due to the occurrence of mutations ”, comments Jorge Sampaio, professor of Clinical Microbiology at the Faculty of Pharmaceutical Sciences at the University of São Paulo and physician responsible for the Sector Microbiology of Fleury Medicina Diagnóstica.
Being PHE licensed is another recognition of Controllab‘s technical competence, since this process validates the representative’s technical and structural capacity to reproduce with the same quality as the licensing brand.
The benefit of the initiative reaches, in addition to Brazil, countries such as Argentina, Bolivia, Chile, Colombia, Ecuador, Paraguay, Peru, Suriname, Uruguay and Venezuela. Access the Controllab website and learn more about the strains available at the MRC Referential Qualitative Cultures and at the STRAINS CONTROL Program, which allow the laboratory more time to dedicate itself to exam analysis with a more practical quality control and with reduced use of resources of the infrastructure.